Germanium and Silicon are two important materials used in electronic devices. They are known as intrinsic material Semiconductor. The germanium atoms has a total of 32 electrons of which 28 are tightly bound to the nucleus and 4 are valence electrons.
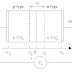
The tightly bound electrons do not leave the nucleus. Therefore, each nucleus and it’s tightly bound electrons can be represented by a circle as shown in a two dimensional representation in Fig 1.1. Each positive charge shown as +4 depicts the nucleus along with tightly bound electrons (Since 4 valence electrons have been taken out, a charge of +4, measured in units of electronic charge remains in each circle). Since both germanium and silicon have 4 valence electrons, this representation is same for both.
Fig.1.1: Two dimensional representation of a crystal of intrinsic semiconductor
Each atom shares its 4 electron with four neighboring atoms and also shares one electron from each of these four neighboring atoms. These shared valence electrons shown by lines in Fig. 1.1. Thus each atom fills its valence orbit with 8 electrons, out of which four are it’s own and four belongs to the neighboring atoms. This forms the covalent bond between atoms. At “0 Kelvin” Temperature there are no free electrons and hence no conductivity. At higher temperatures some of these valence electrons get thermally excited and break the covalent bond. At room temperature the number of such electrons is very small and conductivity is low. The energy required to break the covalent bond is 0.72 ev for germanium and 1.1 ev for silicon. These dislodged electrons are free to move in a random fashion throughout the crystal.
Fig. 1.2 shows the apparent motion of holes due to recombination of holes and electrons. Let an electron breaks its covalent bond at position A and drift to position B under the influences of electron field. If a hole existed at position B, the electron would combine with this hole to neutralize the charge. Now there is a hole at position A and no hole in position B. Thus the hole has moved from position B to A. The drift velocity of electrons and holes is proportional to electric field strength. Thermal agitation produces new electron-hole pairs.
Fig.1.2: Motion of holes in intrinsic semiconductor







0 on: "Intrinsic Semiconductors"